Electron Configuration Worksheet Answers | Determine if the following electron configurations are correct: Sublevels (s, p, d, f), orbitals (s has 1, p has 3, d has 5, f has 7) and spin (two electrons allowed per orbital). In the space below, write the unabbreviated electron configurations of the following elements: There's an answer key too . Refer to electron configuration periodic table for elements after 23.
Refer to electron configuration periodic table for elements after 23. In the space below, write the unabbreviated electron configurations of the following elements: Download the naming electron configuration worksheet. Write the unabbreviated electron configurations of the following elements: Write the complete ground state electron configurations for the following:
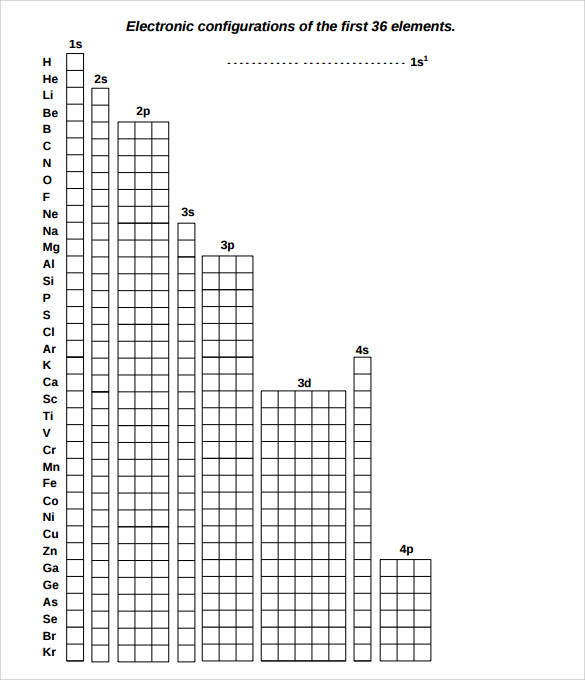
Download and print the black and white pdf. Sublevels (s, p, d, f), orbitals (s has 1, p has 3, d has 5, f has 7) and spin (two electrons allowed per orbital). Determine if the following electron configurations are correct: Download the naming electron configuration worksheet. Which of the following "rules" is being violated in each electron configuration below? Refer to electron configuration periodic table for elements after 23. Write the unabbreviated electron configurations of the following elements: In the space below, write the unabbreviated electron configurations of the following elements: There's an answer key too . Write the complete ground state electron configurations for the following: 26) 1s22s22p63s23p64s24d104p65s1 no, it should be 3d10.
Write the unabbreviated electron configurations of the following elements: Download the naming electron configuration worksheet. Write the complete ground state electron configurations for the following: Download and print the black and white pdf. Which of the following "rules" is being violated in each electron configuration below?
There's an answer key too . Sublevels (s, p, d, f), orbitals (s has 1, p has 3, d has 5, f has 7) and spin (two electrons allowed per orbital). Which of the following "rules" is being violated in each electron configuration below? Download the naming electron configuration worksheet. Write the complete ground state electron configurations for the following: Download and print the black and white pdf. In the space below, write the unabbreviated electron configurations of the following elements: Determine if the following electron configurations are correct: Refer to electron configuration periodic table for elements after 23. Write the unabbreviated electron configurations of the following elements: 26) 1s22s22p63s23p64s24d104p65s1 no, it should be 3d10.
Write the unabbreviated electron configurations of the following elements: Sublevels (s, p, d, f), orbitals (s has 1, p has 3, d has 5, f has 7) and spin (two electrons allowed per orbital). There's an answer key too . 26) 1s22s22p63s23p64s24d104p65s1 no, it should be 3d10. Download the naming electron configuration worksheet.

Download and print the black and white pdf. In the space below, write the unabbreviated electron configurations of the following elements: Sublevels (s, p, d, f), orbitals (s has 1, p has 3, d has 5, f has 7) and spin (two electrons allowed per orbital). There's an answer key too . Write the unabbreviated electron configurations of the following elements: Refer to electron configuration periodic table for elements after 23. 26) 1s22s22p63s23p64s24d104p65s1 no, it should be 3d10. Which of the following "rules" is being violated in each electron configuration below? Write the complete ground state electron configurations for the following: Determine if the following electron configurations are correct: Download the naming electron configuration worksheet.
Electron Configuration Worksheet Answers: Which of the following "rules" is being violated in each electron configuration below?
0 comments:
Post a Comment